Alpha particle scattering experiment class 11 and 12
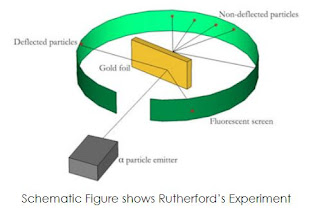
In this experiment, a stream of particles from a radioactive source were directed onto a piece of gold foil that was extremely thin (less than 0.00004 cm thick). The alpha particles were predicted by Thomson's model to simply pass through the gold foil and be identified by a photographic plate positioned behind the foil. However, the experiment's real findings were extremely unexpected. According to observations:
I The majority of the -particles cut through the gold foil unimpeded.
(ii) A few of the -particles had minor angles of deflection.
(iii) A few particles experienced sharp deflections.
(iv) Approximately 1 in 12000 particles rebounded
In 1911, Rutherford provided an explanation of the -ray scattering experiment's findings and put out a different atomic model. According to Rutherford's theory, an atom has a compact, positively charged region at its core that is known as the nucleus. The nucleus is where an atom stores the majority of its mass as well as all of its positive charge. An atom's remaining space must be unoccupied and home to the smaller, negatively charged electrons.
The experimental results from the scattering experiment might be explained using the suggested model. The electron-region particles going through the atom would move straight through with no deflection. Only particles that are in close proximity to the positively charged nucleus have their paths diverted. There are very few -particles that would experience a rebound when they collide with the nucleus. Rutherford was able to forecast the nucleus' size using his model. According to his calculations, the radius of the atom's nucleus is at least 1000 times less than its radius.
Read more Class 11 Chemistry notes on the Rutherford Model Of Atom
Comments
Post a Comment
Thank you we will contact ASAP.